Approach Considerations
Over the past decade, developments in diagnostic techniques have led to a significant improvement in the ability to detect viruses in the respiratory tract. However, the detection of viral pathogens does not always indicate active disease. For example, herpesviruses may become reactivated without causing significant active disease. Similarly, respiratory syncytial virus (RSV) and cytomegalovirus (CMV) can be detected in the presence of other known bacterial pathogens.
In most circumstances, virologic tests are the mainstay of precise etiologic diagnosis. Rapid antigen detection kits can provide results within hours, making them useful in the emergency department. The sensitivity and specificity of these kits varies between 80% and 95%. See Table 1.
Table 1. Diagnostic Techniques Used for Viral Pneumonia (Open Table in a new window)
Virus |
Viral Culture |
Cytologic Evaluation |
Rapid Antigen Detection |
Gene Amplification |
Influenza virus |
HAa, SVb |
|
IFc, ELISAd |
RT-PCRe |
Adenovirus |
CEf, SV |
Intranuclear inclusions |
IF, ELISA |
RT-PCR |
Paramyxoviruses |
||||
Respiratory syncytial virus |
CE, SV |
Eosinophilic cytoplasmic inclusions |
IF, ELISA |
RT-PCR |
Parainfluenza virus |
HA, SV |
Eosinophilic intranuclear inclusions |
IF, ELISA |
RT-PCR |
Measles virus |
HA |
|
|
|
Herpes viruses |
||||
Herpes simplex virus |
CE, SV |
Cytoplasmic inclusions |
IF, ELISA |
PCR |
Varicella-zoster virus |
CE |
Cytoplasmic inclusions |
IF |
RT-PCR |
Cytomegalovirus |
CE, SV |
"Owl's eye" cells |
IF, ELISA |
RT-PCR |
Hantavirus |
|
|
Antibodies against FCVg |
FVC RNA by RT-PCR |
a HA - Hemaglutination b SV - Shell viral culture c IF - Immunofluorescence d ELISA - Enzyme-linked immunosorbent assay eRT-PCR - Reverse transcriptase polymerase chain reaction fCE - Cytopathogenic effects gFCV - Four corners virus |
||||
Viral cultures are still the criterion standard for most viral pathogens, but they take a long time to complete. Therefore, methods faster than this have been introduced. Viral-antigen detection is one of the new tests, but the results are generally less sensitive and less specific than those of conventional cell cultures.
Viral nucleic material amplification, such as hybridizations, various polymerase chain reactions (PCRs), [65] and serologic tests, can be used to follow the increase in specific serum antibodies and for diagnostic purposes.
Recent interest has focused on developing PCR-based tests with single, multiplex, and real-time readings. These tests have sensitivity better than that of cultures.
Nested PCR and reverse-transcriptase (RT) PCR are the most sensitive methods. They increase the detection rate of respiratory viruses in adults with hematologic cancers and pneumonia from 19% to 35%.
PCR is limited by the fact that the results cannot completely rule out contamination of the specimens. In some immunocompromised patients, who shed the virus for long periods, the diagnosis can be of little clinical significance. This limitation is overcome by using quantitative PCR, which shows the level of viral load. The findings can also help in differentiating active infection from contamination.
Because of the difficulty in distinguishing between the various etiologic agents, both viral and bacterial, causing pneumonia, the workup for symptomatic patients with an infiltrate on chest radiograph should include laboratory studies.
Cytologic Evaluation
Respiratory secretions, bronchoalveolar lavage samples, and tissue specimens can be examined using cytologic and histologic techniques. Intranuclear inclusions often exist in cells infected with DNA viruses. Cytoplasmic inclusions usually are present in cells infected with RNA viruses.
CMV infection characteristically is associated with "owl's-eye" cells, which are large cells with basophilic intranuclear inclusions and a surrounding clear zone.
The presence of viral inclusions is diagnostic, although this method has low sensitivity. Therefore, absence of inclusions does not always exclude infection or active disease.
Viral Culture
Viral pneumonia can be diagnosed by isolation and identification of the pathogen through viral culture.
Tissue from the upper or lower respiratory tract, sputum samples, and samples obtained by nasopharyngeal washing, bronchoalveolar lavage, and biopsy may be submitted for viral culture. The use of an appropriate viral transport medium is required. This consists of enriched broth containing antibiotics and a protein substrate.
Viral cultures are performed on various cell lines (eg, monkey kidney cells, diploid fibroblasts). The cell cultures are incubated at 35°C and are examined microscopically on alternate days for an incubation period of 14 days.
The cultures are examined for cytopathogenic effects and for evidence of viral growth. The cytopathogenic effect is the formation of syncytial collections of multinucleated giant cells and rarely is virus specific. Viral growth is detected through hemadsorption testing by demonstrating adherence of red blood cells to the cultured cell monolayer of infected tissue.
Further identification of viruses is accomplished using immunofluorescence (direct or indirect) methods or nucleic acid probes. These techniques are used to identify the specific virus in cell cultures.
Viral cultures are of lower yield in RSV infection (viral lability, lower titers in samples), human metapneumovirus (hMPV) infection, and coronavirus infection (special growth requirements).
Modified cell culture methods called shell vial culture systems are able to detect certain slow-growing viruses. Shell vial culture systems are used widely for earlier detection of CMV, RSV, herpes simplex virus (HSV), adenovirus, influenza viruses, parainfluenza virus (PIV), and other viral pathogens.
In this technique, the prepared clinical specimens are inoculated on to adherent cell monolayers grown on round coverslips in small vials. The vials are centrifuged at low speed for one hour, after which fresh culture medium is added. Next, the vials are incubated and examined serially to detect viral antigen or DNA expression. Results typically become available in 2-3 weeks.
Sputum Gram stains
Sputum Gram stains are often contaminated with oral pathogens and are difficult to obtain. They are not recommended by the American Thoracic Society or the American College of Emergency Physicians, although the Infectious Diseases Society of America recommends obtaining a sputum sample, particularly in hospitalized patients.
Blood cultures
The utility of blood cultures in patients with pneumonia remains controversial. Local hospital protocols should be consulted to determine which patients with pneumonia are candidates for hospitalization and who should have blood cultures drawn prior to administration of medications.
Rapid Antigen Detection
Rapid antigen detection tests provide faster results because the test is performed directly on specimens obtained from patients. Nasal swabs or washings are easy to obtain
Immunofluorescence assay and enzyme-linked immunosorbent assay (ELISA) are available for the diagnosis of HSV, RSV, influenza viruses A and B, PIV, CMV, and other respiratory viruses. ELISA can detect viral antigens, while an immunofluorescence assay requires the presence of prepared, intact, infected cells. The sensitivity and specificity of these methods varies depending on the virus being sought and the particular diagnostic assay being used.
The advantages of antigen detection tests are higher specificity for individual viruses. Furthermore, these assays remain positive for several days to weeks, long after the culture technique can detect viable virus.
The disadvantages of these methods are that the overall sensitivity is lower than that of viral cultures. Therefore, antigen detection methods should be used in conjunction with cell culture for optimal diagnosis of viral infections. [66]
RSV rapid antigen detection is useful in young children, who shed high titers of virus, but sensitivity is low in adults (0-20%) when compared with RT-PCR.
Sensitivity for seasonal influenza in adults ranges between 50% and 60%, and specificity is greater than 90%. Novel swine-origin influenza A H1N1 virus should be detectable by rapid influenza testing. However, sensitivity with rapid tests is significantly lower (51-63%) when compared with RT-PCR. Rapid influenza tests unfortunately have very poor sensitivity and specificity for the avian H5N1 influenza virus and are therefore not recommended.
With ED patients, a call to the hospital laboratory is suggested to determine the optimal test to be ordered and whether a specific viral identification should be requested or whether a general request for viral detection will result in testing for a panel of pathogens. If rapid test results are negative but clinical suspicion is high, cultures can be obtained and the patient treated until results are known. Positive viral identification cannot rule out bacterial co-infection.
Gene Amplification
PCR is a highly sensitive and specific technique for amplifying genes to detect the presence of a virus. For many viruses, this is the diagnostic test of choice, and if possible, it should be used in combination with viral culture and immunocytologic and rapid antigen detection. PCR technology allowed the discovery of such viruses as RSV, hMPV, and coronaviruses in causing pneumonias.
For influenza H1N1 and avian influenza, RT-PCR of either nasopharyngeal swabs or bronchial aspirates/sputa is the diagnostic modality of choice.
PCR has become especially useful for the detection of CMV in various body fluids (eg, blood, urine) in severely immunocompromised patients, particularly hematopoietic stem cell transplant (HSCT) recipients.
A newly developed molecular diagnostic technique, multiplex reverse transcriptase polymerase chain reaction (MRT-PCR), permits rapid detection of influenza virus types A and B, RSV (types A and B), adenoviruses, PIV (types 1, 2, and 3), hMPV, and rhinovirus in appropriate respiratory tract secretions. [67, 68] The single-step MRT-PCR technique has high sensitivity and specificity. Influenza H1N1 is reported as "non-typeable influenza" by the MRT-PCR.
Serologies
Almost all viral infections can be diagnosed via paired acute/convalescent serologies (usually measured by complement fixation or enzyme immunoassay [EIA]). Because many of these viruses are ubiquitous, this method ideally requires a four-fold rise in titers.
Because, by definition, this technique requires blood to be drawn in the convalescent phase, it is not as useful in the acute management of the patient, unless enough time has elapsed such that the acute draw might represent the convalescent titer. In this situation, one high titer may give the diagnosis.
Serologies are particularly useful for definitively confirming the diagnosis, especially the positive results of other diagnostic tests.
Arterial blood gases
ABGs may be of great value in identifying hypoxemia in severe disease but are unnecessary in mild or moderate disease. Pulse oximetry should be obtained in all patients.
Virus-Specific Laboratory Studies
Virus-specific laboratory studies exist for viruses such as influenza virus, respiratory syncytial virus, adenoviruses, parainfluenza virus, human metapneumovirus, varicella-zoster virus, measles virus, cytomegalovirus, herpes simplex virus, and hantavirus. Renal function should be checked and followed in patients with serious 2009 H1N1 disease because acute renal injury is common in such patients. [69, 70]
Influenza virus
Influenza can be isolated from nasal/throat swabs, nasal washes, and sputa on viral culture (in a variety of kidney cell lines). Throat swabs have the lowest yield. Ninety percent of positive cultures can be detected within three days of inoculation, and the remainder can be detected by day seven.
Many rapid tests exist for influenza virus types A and B, but sensitivities are 40-80% when compared with viral culture. However, the specificity is high (85-100%). Per above, novel S-OIV can be detected by rapid tests, but the sensitivity is low (51-63%), and for avian influenza, this test is not useful. RT-PCR of sputa is the most useful diagnostic test for the latter 2 influenza strains.
Respiratory syncytial virus
RSV can be isolated via culture (HEp-2, HeLa, A549 cell lines), whereby nasopharyngeal washes or tracheal secretions are of higher yield than nasal swabs. For immunocompromised hosts, 15% of nasopharyngeal wash specimens are positive, compared with 71% of endotracheal secretions and 89% of bronchioalveolar washes.
Rapid detection by EIA has a sensitivity of 50-90% (higher in children but lower in adults), but specificity is high (90-95%). RT-PCR is also available, especially in combination with primers of other viruses (such as MRT-PCR).
Adenoviruses
Adenoviruses can be isolated from respiratory secretions and can be grown in human embryonic kidney cells, human laryngeal tumor cells (HEp-2), and HeLa cells. Cytopathic effects appear in 2-20 days and include eosinophilic and diffuse basophilic intranuclear inclusions.
Serotype 14 can be diagnosed by viral culture, direct fluorescent antibody rapid antigen detection, and PCR. In the HSCT population, adenovirus PCR from plasma has been shown to be a good predictor of disease, especially at a threshold of 1000 copies/mL. [71]
If adenovirus 14 is suspected, because of severity of illness and negative bacterial and viral cultures, clinicians should contact their state public health department for aid in testing. [72]
Parainfluenza virus
Parainfluenza can be isolated in cell culture (various kidney cell lines), preferably from nasal secretions. Isolation of this virus is strong evidence of its infection. PCR is more sensitive and rapid for detection and is also available in the single multiplex assay.
Human metapneumovirus
It is difficult to isolate hMPV in standard cell cultures, and it replicates (grows) very slowly. The optimal cell line is tertiary cynomolgus monkey kidney or rhesus monkey kidney cell culture with trypsin added; cultures need to be observed for 21 days for cytopathic effect. Because of culture difficulties, RT-PCR is the preferred method for diagnosis.
Varicella-zoster virus
Varicella-zoster virus (VZV) infection and pneumonia can be diagnosed mostly on clinical grounds. VZV can be isolated from vesicular fluid, respiratory secretions, or cerebrospinal fluid (CSF) by culture. Rapid antigen detection tests such as direct immunofluorescence can be performed on cell scrapings of skin lesions.
A Tzanck smear obtained by unroofing a cutaneous lesion for material may show multinucleated giant cells with eosinophilic intranuclear inclusions but cannot distinguish between HSV and VZV and has a sensitivity of 60%. VZV PCR can also be performed using many different body fluids (particularly CSF).
Measles virus
Measles pneumonia is usually diagnosed clinically, but laboratory diagnosis can be helpful. Measles virus can be grown in monkey and human kidney cell lines. Cytopathic effects are observed in 6-10 days. Eosinophilic inclusions are found in the cytoplasm and nucleus of the infected cells. Immunofluorescent examination of cells from nasal exudates can also be used. Paired acute/convalescent serologies are also used for diagnosis.
Cytomegalovirus
CMV pneumonia is diagnosed on the basis of clinical presentation in the appropriate host (severely immunocompromised patient) and histopathologic findings (owl's eyes basophilic intranuclear inclusions) on lung biopsy tissue. Blood CMV PCR and/or CMV blood culture positivity lends further support for the diagnosis. However, positive CMV cultures and/or PCR should be interpreted in view of other evidence of disease, because asymptomatic shedding can occur in saliva, sputa, blood, urine, and other body fluids.
Documentation of viremia by shell vial culture technique correlates with positive CMV blood cultures and usually signifies CMV disease, with the above caveats. Presence of pp65 antigenemia also correlates with the development of CMV disease in the HSCT population.
Herpes simplex virus
HSV pneumonia can be confirmed in the appropriate host (ie, a severely immunocompromised patient) using lower respiratory tract viral culture (preferably bronchoscopy specimen) and histology of pulmonary tissue showing multinucleated giant cells. Antigen detection and PCR of sputa are overly sensitive (false positives), and serologies are not useful for this type of pneumonia.
Hantavirus
Hantavirus infection is based on serum detection of hantavirus–specific antibody and, for the majority of the United States, on the development of Sin Nombre Virus (SNV) specific antibodies. Less commonly, in a more research-oriented setting, reverse transcriptase polymerase chain reaction (RT-PCR) can be used to detect hantavirus RNA in peripheral blood mononuclear cells, lung tissue, and/or red blood cells. The serodiagnosis of acute hantavirus infection is confirmed by detecting the genetic material in peripheral blood mononuclear cell preparations with RT-PCR.
Middle East respiratory syndrome coronavirus
The first US case of MERS-CoV was confirmed on May 2, 2014. [73] The Centers for Disease Control and Prevention (CDC) has issued the following recommendations for the evaluation and control of Middle East respiratory syndrome coronavirus (MERS-CoV) in the United States [74, 75] :
-
Testing for MERS-CoV can be performed simultaneously with testing for other respiratory pathogens
-
Confirming the presence of another respiratory pathogen does not rule out the need to test for MERS-CoV in patients who develop fever and pneumonia or acute respiratory distress syndrome (ARDS) within 14 days after leaving the Arabian Peninsula or nearby regions or following close contact with someone with fever and ARDS who recently had been to this area
-
If ARDS occurs in a cluster of patients, tests for common respiratory pathogens should be performed and the cases reported to local and state public health departments. If health-care providers are in the cluster and the cause of ARDS remains unexplained, patients should be considered for MERS-CoV testing even if travel-related disease exposure has been ruled out
-
Laboratory confirmation of MERS-CoV requires a positive PCR assay of 2 or more specific genomic targets or 1 positive target with sequencing of a second; even if an etiology other than that for MERS-CoV is found, a patient whose disorder meets these requirements can still be classified as a probable MERS-CoV case
-
Travelers to Saudi Arabia should avoid contact with ill persons, wash their hands often, and seek medical care if they develop fever with cough or shortness of breath during their trip or within 14 days after returning home
-
Standard, contact, and airborne precautions are recommended for hospitalized patients with confirmed or suspected MERS-CoV infection
-
Federal isolation and quarantine are authorized for patients with confirmed or probable MERS-CoV infection until they are no longer contagious
-
See Middle East Respiratory Syndrome (MERS) for more information.
Chest Radiology
Chest radiography usually demonstrates bilateral lung involvement (as opposed to the lobar involvement commonly seen with bacteria), but some features are characteristic of individual viruses.
For more information, see Imaging in Viral Pneumonia.
Influenza pneumonia
Radiographic findings in influenza pneumonia are similar to those described for other respiratory viral infections. Perihilar and peribronchial infiltrates occur commonly, while progression to diffuse interstitial infiltrates is observed with severe disease.
Avian influenza pneumonia
Avian influenza (H5N1) radiographic findings have included patchy, interstitial, and/or diffuse infiltrates, consolidation, pleural effusion, and pneumothorax. Some have progressed to acute respiratory distress syndrome (ARDS). [52]
S-OIV pneumonia
Novel S-OIV cases have primarily presented with bilateral patchy alveolar opacities, with a preference for basal sections, and interstitial opacities. [3]
RSV pneumonia
RSV pneumonia typically presents with patchy bilateral alveolar infiltrates and interstitial changes (similar to influenza). Reports of culture-proven cases of RSV pneumonia presenting with small, ill-defined infiltrates have been described. [20, 53]
Adenovirus pneumonia
Adenovirus pneumonia usually presents with diffuse, bilateral and patchy, ground-glass infiltrates with a preference for lower lobes, although it can present with lobar consolidation, which is rarer among viral pneumonias. [4] Radiographic presentation for the serotype 14 outbreak in Oregon included single-lobe infiltrates (54%), multilobe infiltrates (38%), interstitial infiltrates (12%), and pleural effusion (15%). Among those with single-lobe infiltrates, 71% progressed to multilobe involvement. [16]
PIV pneumonia
In PIV, the "steeple sign" (progressive subglottic narrowing), classic for croup in children, is rarely seen in adults. Instead, chest radiographs may reveal findings ranging from focal infection to diffuse interstitial infiltrates or diffuse mixed alveolar-interstitial infiltrates consistent with acute lung injury. CT scan findings of six HSCT recipients with PIV (type 3) diagnosed as the sole etiologic pathogen revealed multiple small nodules (diameter, < 5 mm) without cavitation in a peribronchial distribution. [5]
hMPV pneumonia
In an outbreak of hMPV pneumonia, radiographic findings were bilateral, interstitial, and alveolar infiltration in 43% and unilateral infiltration in 57%. [50] In HSCT recipients, CT scans have shown bilateral nodular and extensive infiltrates and pleural effusion. [49]
Coronavirus pneumonia
Coronavirus pneumonia, including SARS, typically shows ground-glass opacities and focal consolidations, especially in the periphery and subpleural regions of the lower zones. Progressive involvement of both lungs is common. In SARS, shifting of radiographic shadows and spontaneous pneumomediastinum have been seen. [55]
VZV pneumonia
In VZV pneumonia, radiographic findings are diffuse, fluffy, reticular or nodular infiltrates that can be rapidly progressive. Pleural effusion and peripheral adenopathy can occur. Radiographic abnormalities are more prominent during the peak of the rash and resolve rapidly with clinical improvement. Long-term respiratory sequelae are infrequent in survivors, although small, diffusely scattered, punctate lung calcifications may persist on chest films
CMV pneumonia
The two patterns of CMV involvement include (1) a multifocal or miliary pattern characterized by discrete spherical lesions as large as 4 mm in diameter, with alveolar hemorrhage, fibrin deposition, and a moderate neutrophilic response; and (2) a diffuse interstitial pneumonitis with interstitial edema, varying degrees of fibrosis, lymphoid cell infiltration, and alveolar-cell hyperplasia. (See the images below.)
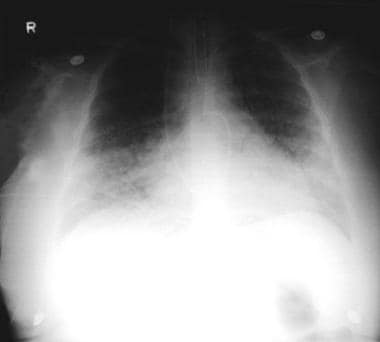 Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus.
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus.
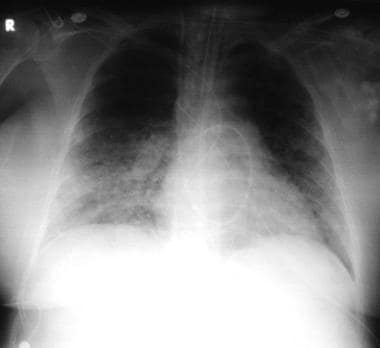 Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus. She was treated with acyclovir; resolution of varicella-zoster virus infection occurred after 7 days of therapy.
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus. She was treated with acyclovir; resolution of varicella-zoster virus infection occurred after 7 days of therapy.
In CMV pneumonia, chest radiographs show interstitial infiltrates predominantly in the lower lobes. Advancement to diffuse, interstitial infiltrates is observed in patients with organ transplantation. The most common CT scan findings are a combination of multiple, small centrilobular nodules (55-99%), patchy ground-glass opacities (44-100%), and small bilateral/asymmetric foci of consolidation (44-70%). [39]
HSV pneumonia
HSV can produce focal lesions on chest radiographs that begin as small centrilobular nodules and patchy ground-glass opacities and consolidation. As the disease progresses, the nodules coalesce to form extensive infiltrates. [39]
Hantavirus pneumonia
Hantavirus infection may show a normal chest radiograph during early disease. This is followed by signs of interstitial edema, Kerley B lines, peribronchial cuffing, and indistinct hila. Progression to the pulmonary edema phase over the subsequent 48 hours is indicated by centrally located dense alveolar infiltrates, unlike the more peripheral infiltrates of ARDS from other causes. With further progression, pleural effusions also may develop.
Guidelines
No firm guidelines exist for when to obtain a chest radiograph in patients to aid in diagnosing lower respiratory tract infection. Chest pain, dyspnea, and productive cough are some of the indications used by clinicians.
The Infectious Diseases Society of America recommends chest radiography to confirm infiltrates when pneumonia is suspected for the following reasons: the severity of disease may be revealed, detection of pneumonia may not be possible on purely clinical grounds, and antibiotics are not useful for treatment of bronchitis. It is recommended that a chest radiograph be obtained in patients with suspected pneumonia, both to find complications, such as pleural effusions, and to discourage the use of antibiotics in healthy patients with bronchitis rather than pneumonia.
Antibiotics are recommended for pneumonia, and a chest radiograph is necessary to make this diagnosis. Antibiotics have not been shown to be efficacious in bronchitis. The widespread use of antibiotics in inappropriate situations is leading to drug resistance and may explain the increases in death rates since 1979. Antibiotics can cause adverse drug reactions. Thus, antibiotics should be avoided when they are not needed. However, if an infiltrate is seen on a chest radiograph, it may be due to viral or bacterial disease or both. In the ED, differentiating the etiology may be impossible.
None of the viral etiologies of pneumonia result in pathognomonic findings on chest radiographs, and bacterial pneumonia cannot be differentiated from viral pneumonia based on radiographic findings. Of concern was the fact that some patients with SARS had negative findings on chest radiographs but infiltrates were seen on chest CT. Chest radiography may reveal the following findings:
-
Patchy interstitial or alveolar infiltrate, which may be bilateral or involve 2 or more lobes
-
Peribronchial thickening
-
Consolidation
-
Pleural effusion
For more information, see Imaging in Viral Pneumonia.
Lung Biopsy
Infrequently, lung biopsy (ie, transbronchial via a bronchoscope, transthoracic via a thoracoscope, or open lung) is required to make a diagnosis in very ill patients, who often are immunocompromised.
Bronchiolar lavage
Bronchiolar lavage may be useful to obtain material for cytopathologic analysis and microbiologic studies.
Histologic Findings
In general, when viruses cause pneumonia, it initially affects the parenchyma adjacent to terminal and respiratory bronchioles and subsequently progresses to involve the entire lobule. With rapidly progressive pneumonia, diffuse alveolar damage is seen, consisting of intra-alveolar hemorrhage, interstitial lymphocyte infiltration, edema, fibrin deposition, type 2 pneumocyte hyperplasia, and formation of hyaline membranes. [76]
Influenza and avian influenza pneumonias
Influenza histopathology of lung tissue reveals edema, focal hemorrhages, and cellular infiltration. Alveoli may be denuded of epithelium, and intra-alveolar hemorrhage is common. The presence of an acellular hyaline membrane lining the alveoli is typical of influenza pneumonia.
Avian influenza (H5N1) typically shows fulminant, necrotizing, diffuse alveolar damage with patchy, interstitial, paucicellular fibrosis. (H1N1 pneumonia has shown diffuse alveolar damage, thick hyaline membranes, and prominent fibroblast proliferation.)
Varicella-zoster, measles, and CMV pneumonias
Varicella-zoster pneumonia shows focal necrosis, consolidation, a mononuclear infiltrate, and intranuclear inclusion bodies.
Measles pneumonia has been called Hecht giant cell pneumonia because a predominantly interstitial infiltrate with mononuclear cells and multinucleated giant cells is present on histology.
CMV pneumonia histopathology demonstrates typical cytomegalic cells with intranuclear and cytoplasmic inclusions. Histopathologic examination of lung tissue shows mononuclear interstitial infiltrates, thickened alveolar walls, fibrinous exudates, and hemorrhage. The cells containing inclusion bodies can be difficult to detect in mild cases.
HSV and hantavirus pneumonias
HSV pneumonia causes parenchymal necrosis, hemorrhage, and mononuclear infiltrates. Upon bronchoscopy, one may observe trachitis, bronchitis, and typical punctate mucosal lesions. Pathology findings in HSV infection show multinucleated giant cells and intranuclear inclusions.
Hantavirus pneumonia histopathology reveals interstitial infiltrates of T lymphocytes and alveolar pulmonary edema without marked necrosis or polymorphonuclear leukocyte involvement. This finding is consistent with the pathogenesis being mainly caused by vascular permeability increase, via an immunopathologic mechanism. [77]
-
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus.
-
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus. She was treated with acyclovir; resolution of varicella-zoster virus infection occurred after 7 days of therapy.
-
Bilateral interstitial infiltrates in a 31-year-old patient with influenza pneumonia.
-
Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
-
High-power Papanicolaou (Pap) stain showing a virus on a bronchial wash. Viruses in general show "smudgy" nuclei, may or may not show nuclear or cytoplasmic inclusions, and may demonstrate other features such as multinucleation or margination of chromatin to the periphery of the cell nucleus. Certain features are more indicative of one virus over another. This is an example of herpes virus (cells infected are located in the center right).
-
High-power Papanicolaou (Pap) stain of cytomegalovirus (center). This cell has a very large, dark intranuclear inclusion.
-
High-power hematoxin-and-eosin stain of giant cell pneumonia following measles. A multinucleated cell (center) contains eosinophilic intranuclear inclusions.
-
High-power hematoxin-and-eosin stain of numerous cells infected with the cytomegalovirus (large cells with enlarged nuclei containing dark-purple intranuclear inclusions surrounded by a clear halo).
-
High-power hematoxin-and-eosin stained herpes simplexvirus, characterized by "smudgy" degenerating nuclei. Some cells are multinucleated with margination of the chromatin to the periphery of the nuclei and molding of the nuclei to each other.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Supportive Care
- Influenza Pneumonia
- Respiratory Syncytial Virus Pneumonia
- Adenovirus Pneumonia
- Parainfluenza Virus Pneumonia
- Human Metapneumovirus Pneumonia
- Coronavirus Pneumonia
- Varicella-Zoster Virus Pneumonia
- Measles Pneumonia
- Cytomegalovirus Pneumonia
- Herpes Simplex Virus Pneumonia
- Hantavirus Pneumonia
- Prevention
- Consultations
- Show All
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References