Reports of patients using standard pen needles to inject insulin without removing the inner needle cover have prompted the US Food and Drug Administration (FDA) to issue a safety communication on proper use of pen needles.
Posted September 27 on the agency's website, the safety notice reminds healthcare providers, patients, and caregivers about the correct use of pen needles and potential risks if the standard pen needle's inner needle cover is not removed before injection.
Pen needles are used to inject different types of medicine with pen injectors. Standard pen needles often have an outer cover and a removable inner needle cover. Both the outer cover and inner needle cover must be removed before an injection.
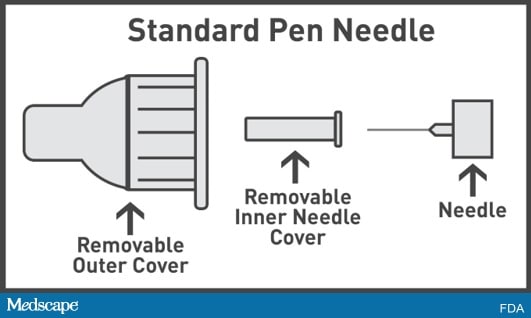
Safety pen needles have an outer cover and a fixed inner needle shield designed to help prevent injury. The outer cover is removed before an injection, but the fixed inner needle shield is not removed before an injection.

The FDA notes that the same pen injector can be used with both standard and safety pen needles and patients may be taught using one type of pen needle, then receive the other type later. "This could cause confusion about how to use the pen needle correctly and may prevent the patient from getting the medicine they need," the FDA cautions.
The FDA says it has become aware of patients using standard pen needles to inject insulin without removing the inner needle cover. In these cases, the inner cover stopped the needle from entering the skin and the patients did not receive the insulin, leading to hyperglycemia in some cases. One patient was hospitalized and died because of prolonged hyperglycemia, according to the FDA.
The FDA recommends health providers take the following actions:
Train and educate patients and caregivers on proper use of pen needles.
Ensure they can demonstrate correct technique to verify proper use of their pen needles.
Be sure they are aware of the different types of pen needles and know which type they use.
For standard pen needles with an outer cover and inner needle cover, remind patients to remove both covers before use.
For safety pen needles, remind patients to remove only the outer cover, as the fixed inner needle shield remains in place.
Explain the signs and symptoms of under-dose (and over-dose) of their medication, how to monitor their medical condition (for example, blood glucose levels), and when to contact their healthcare provider.
The FDA encourages patients and caregivers to check each new box of pen needles to see if they are the same type as the ones they were trained to use, and if not, to ask their healthcare provider to demonstrate proper use.
The FDA has asked pen needle manufacturers to review their instructions for use and training materials to assess the need for updates to clearly explain how to use the pen needle safely. The agency has also requested that standard pen needle manufacturers consider adding a warning to the labeling regarding the need to remove both the outer cover and inner needle cover before use.
Health providers are encouraged to report problems with pen needles to MedWatch, the FDA Safety Information and Adverse Event Reporting program. The report should include the device manufacturer and model of the pen needle; name of the medication being delivered through the pen needle; and a clear description of event or issue and any applicable patient outcomes.
© 2018 WebMD, LLC
Send comments and news tips to news@medscape.net.
Cite this: Possible Pen Needle Confusion Prompts FDA Safety Alert - Medscape - Sep 27, 2018.









Comments