Two different drugs that target the Janus kinase (JAK) pathway have shown promise for alopecia areata (AA), inducing hair growth in a large proportion of participants in two separate small studies.
One of the drugs is ruxolitinib (Jakafi, Incyte Pharmaceutical), a JAK inhibitor indicated for intermediate- or high-risk myelofibrosis, and the other is tofacitinib (Xeljanz, Pfizer), a JAK inhibitor used to treat rheumatoid arthritis.
Both studies have been published in the Journal of Clinical Investigation/Insight.
The ruxolitinib study involved 12 patients with moderate-to-severe AA; three quarters of patients experienced significant hair regrowth, and the responders to the treatment exhibited a 92% reduction in hair loss from baseline.
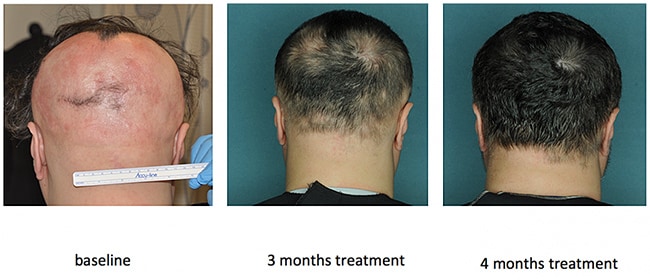
The tofacitinib study had 66 patients with various degrees of AA; almost two thirds (64%) of the cohort responded to treatment, and 32% of patients achieved an improvement of 50% or greater in only 3 months of therapy.
However, the response was not durable, and hair loss began soon after therapy was stopped.
"We feel that tofacitinib therapy for advanced alopecia areata represents an important breakthrough for patients with no alternatives," co-lead author Anthony Oro, MD, PhD, professor of dermatology from Stanford University, California, told Medscape Medical News. "However, at the dose and duration we used, the effect was not long-lasting once the medicine is stopped."
"We are exploring dose, length, and JAK subtype variables and how they affect length of remission," he added. "Through our studies, we hope to not only develop an effective treatment paradigm, but also learn more about this common and puzzling disease."
Promising Findings From Small Study
The ruxolitinib study was conducted by Julian Mackay-Wiggan, MD, MS, associate professor and director of the clinical research unit in dermatology at Columbia University Medical Center in New York City, and colleagues. In this small open-label clinical trial, the 12 patients received oral ruxolitinib, 20 mg twice per day, for 3 to 6 months, and then were followed for 3 months off of treatment.
The primary endpoint was the proportion of patients who experienced 50% or greater hair regrowth from baseline to the end of the treatment period.
Significant hair regrowth was observed in 9 of 12 patients (75%), and the primary outcome of at least 50% regrowth was achieved.
The mean baseline Severity of ALopecia Tool (SALT) score of 65.8% ± 28.0% also declined to 24.8% ± 22.9% at 3 months and continued decreasing to a score of 7.3% ± 13.5% by the end of 6 months of treatment (P < .005).
In addition, 7 of the 9 responders achieved over 95% regrowth by the end of their treatment, with hair regrowth noted as soon as 4 weeks after start of ruxolitinib.
The durability of these responses was assessed during the 3-month follow-up period off treatment. Of the 9 responders, 3 reported that they were shedding hair at week 3 after ruxolitinib was discontinued and that by week 12 they were experiencing marked hair loss.
However, their hair loss did not reach baseline levels, and 6 of the responders also reported that they were experiencing shedding but without major hair loss.
In this cohort, ruxolitinib was generally well tolerated and no serious adverse effects were reported. Side effects of the drug occurred infrequently, and none of the patients required discontinuation of therapy, the researchers noted.
Ruxolitinib is currently used as a cancer therapy, but many diseases may be affected by the same immune pathway, explained Dr Mackay-Wiggan.
"This appears to be the case with myelofibrosis, alopecia areata, rheumatoid arthritis, and other disorders," she told Medscape Medical News.
Courtesy of laboratory of Angela Christiano/Columbia University Medical Center
These findings, along with those for tofacitinib, suggest a possible breakthrough in treating alopecia areata, noted Dr. Mackay-Wiggan. "But much more work needs to be done."
"The next step would be to conduct larger, well-powered, randomized, double-blinded, placebo-controlled trials to verify our results," she said. "Much remains to be ascertained regarding optimal dosing, duration of treatment, optimal maintenance treatment, and long- and short-term safety of the drug in these patients. Alopecia areata is very variable, so length of treatment and optimal maintenance may vary from patient to patient."
But funding is needed to get these trials completed, Dr Mackay-Wiggan said. "That has been a major roadblock to research in alopecia areata in general. Some wonderful companies, including Locks of Love and Alopecia Areata Initiatives, provided funding to allow us to proceed this far, but more will be needed."
Regrowth With Tofacitinib
In the second study, Dr Oro and Brett King, MD, PhD, assistant professor of dermatology from Yale University, New Haven, Connecticut, and their colleagues evaluated the efficacy of tofacitinib in a larger cohort of patients.
The cohort included 66 patients with different clinical subtypes of AA: 11 with AA (16.7%), 3 with ophiasis pattern AA (4.6%), 6 with alopecia totalis (AT) (7.6%), and 46 with alopecia universalis (AU).
Patients received 5 mg of tofacitinib twice daily for 3 months, and the study endpoints included regrowth of scalp hair, as assessed by the SALT scale, duration of hair growth after completion of therapy, and disease transcriptome.
After 3 months, the median percentage change in the severity of SALT score was 21%. Nonresponders had a percentage change in SALT score improvement of less than 5% (36% of patients), intermediate responders saw improvement between 5% and 50% (32% of patients), and strong responders experienced an improvement that was greater than 50% (32% of patients).
When the authors looked at response in the different subtypes, patients with AA had a 34% greater percentage change in SALT score compared with those with AU (P = .0005), and patients with ophiasis had a 48% greater percentage change in SALT score compared with patients with AU (P = .006).
To assess durability of hair regrowth after treatment was completed, follow-up continued for 3 months after drug cessation. A total of 20 patients were available for evaluation, and all of them experienced hair loss at a median of 8.5 weeks.
The researchers noted that few adverse events were noted in this patient population, in contrast to studies of tofacitinib used to treat rheumatoid arthritis and psoriasis, in which transaminitis and infections occurred. In this study, all reported events were limited to grade I and II infections, including paronychia and upper respiratory tract infection, which were reported in 25% of patients.
The ruxolitinib study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. Dr Mackay-Wiggan has disclosed no relevant financial relationships; two coauthors report disclosures.
The tofacitinib study was supported by the US Department of Veterans Affairs Office of Research and Development, National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR47223 and U01 AR67173, the National Psoriasis Foundation, the Swedish Society of Medicine, the Fernström Foundation, the Locks of Love Foundation, the National Alopecia Areata Foundation, and the Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research. Dr Oro has disclosed no relevant financial relationships; two coauthors have disclosures.
JCI Insight. 2016;1:e89790, e89776. Ruxolitinib study abstract, tofacitinib study abstract
Follow Medscape Oncology on Twitter: @MedscapeOnc
Medscape Medical News © 2016
WebMD, LLC
Send comments and news tips to news@medscape.net.
Cite this: JAK Inhibitor Drugs Show Promise in Alopecia Areata - Medscape - Sep 28, 2016.









Comments